It’s prime time to harness the power of RNA & AI
A single platform, diverse applications, unique insights
While cancer therapies have significantly improved patient outcomes,
challenges such as variable patient responses and immune-related side effects persist.

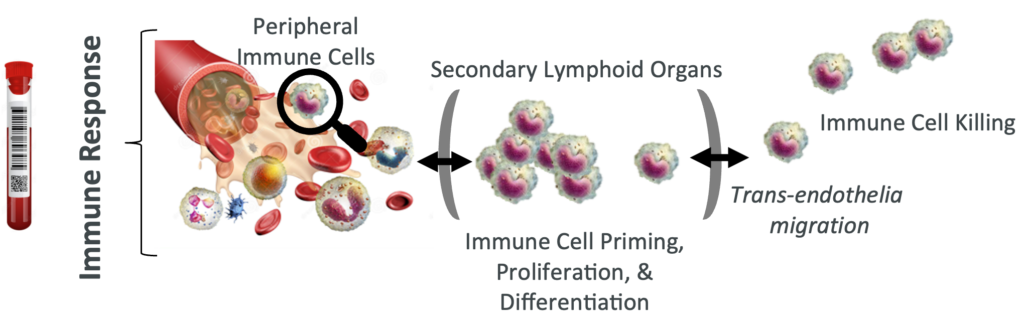
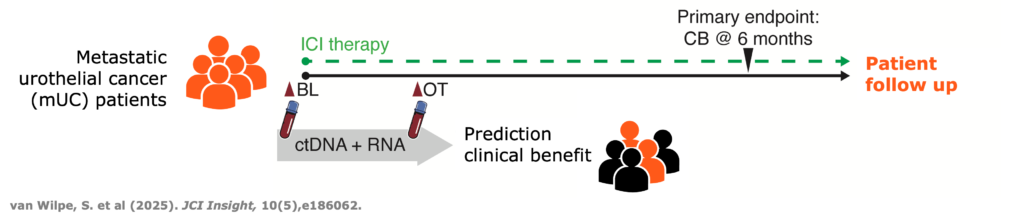
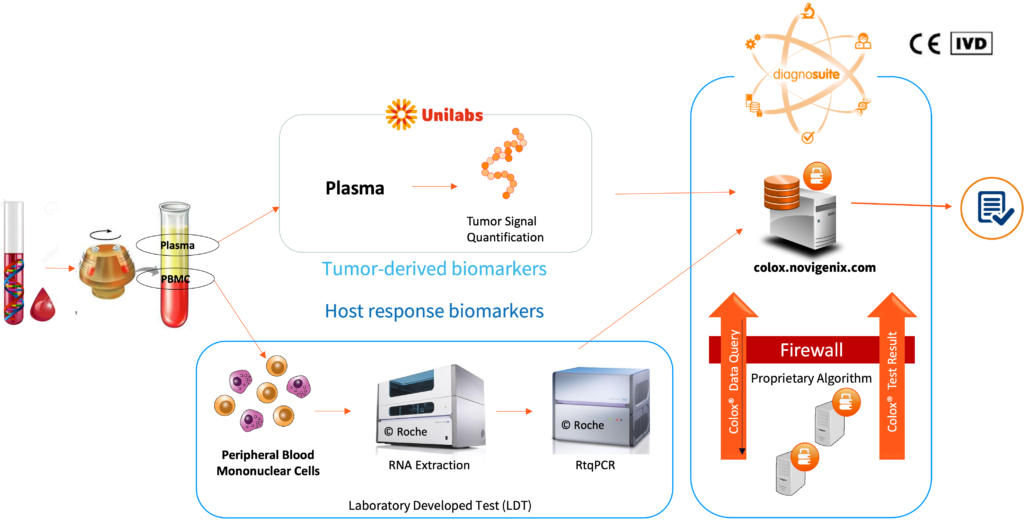
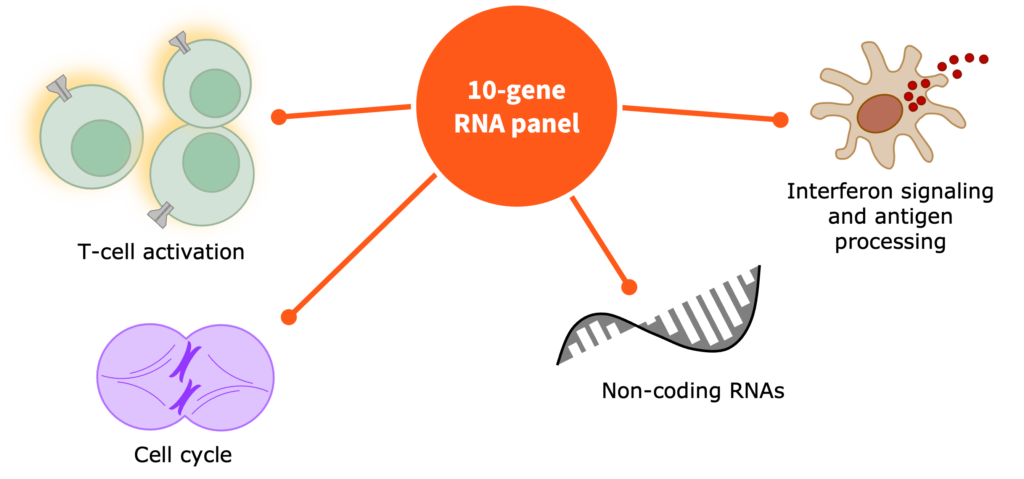
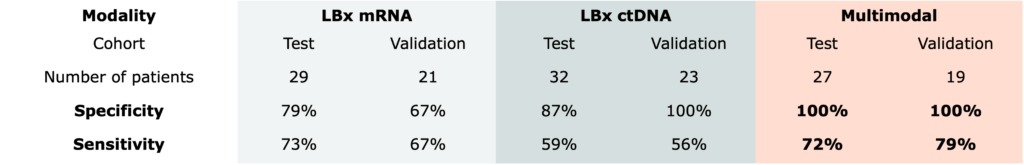
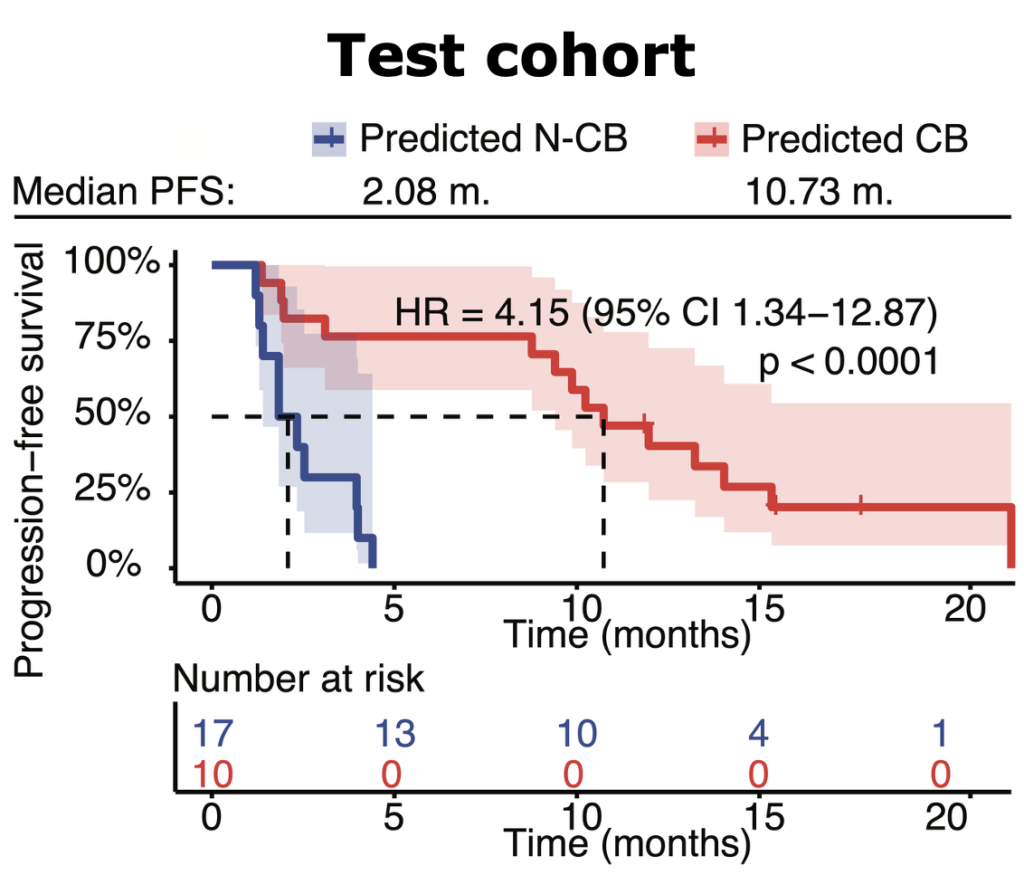
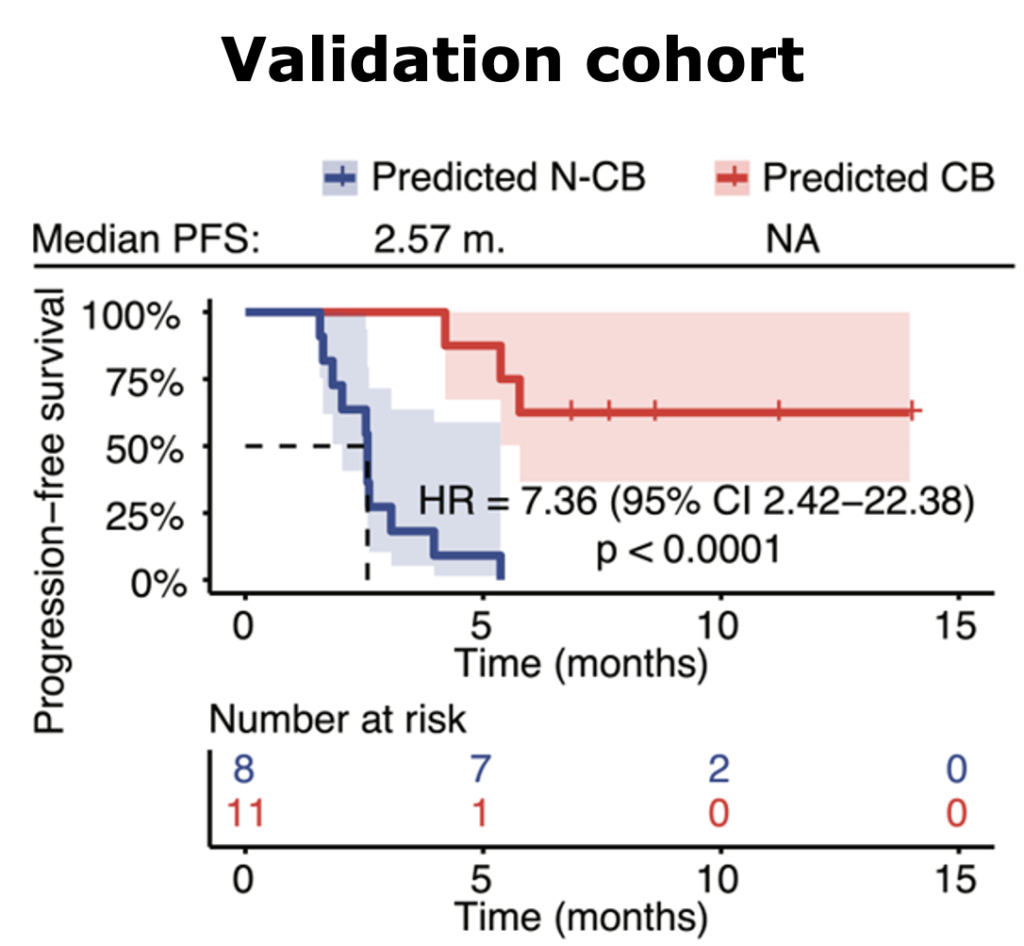
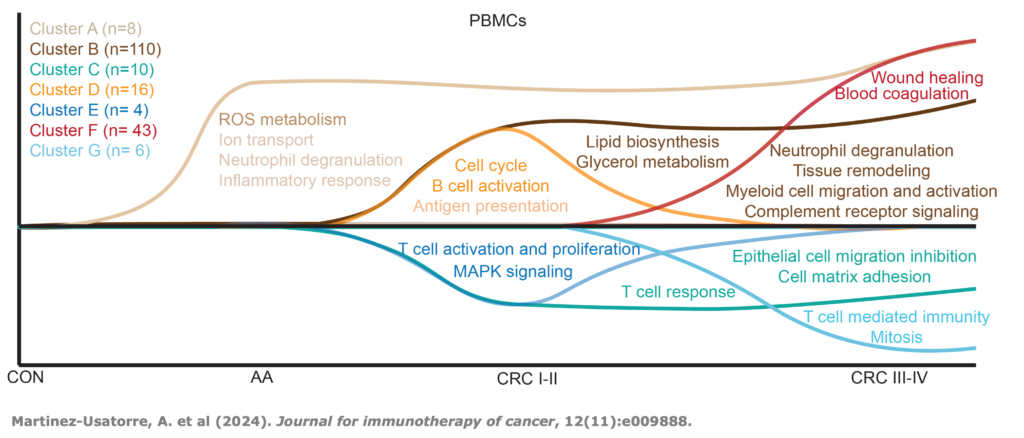
Multiomic integration of LBx RNA immunotranscriptome data and LBxctDNA data for:
Colorectal cancer (CRC) can develop suddenly but progresses very slowly before the first symptoms appear.
The five-year survival rate for early stages of the cancer is over 90%, compared to just 13% for late stages of the cancer when symptoms appear!
Colonoscopy is the recommended standard of care for CRC screening when no symptoms are yet present, however, it is invasive, inconvenient, and expensive resulting in low patient compliance.
Our early cancer detection platform offers: